Research
Latest Research
Prof. Fei Dou's lab' discovers the new regulation mechanism of Hsp90 in the nutrient sensing and aging process
Environmental stresses such as temperature, osmotic pressure, lack of nutrients and reactive oxygen species (ROS) affect biological function. In response, organisms have evolved a wide array of mechanisms to adapt to fluctuations in external conditions. When faced with long-term stress, budding yeast, a model unicellular organism, exits the cell cycle and stays in G0. There are changes to many processes when cells begin their entry into quiescence.
Prof. Fei Dou's lab' is interested in the roles of molecular chaperone Hsp90 under quiescence or starvation conditions. They found that Hsp82 (HSP90 yeast homologue) distributes evenly in the cytoplasm and nucleus in vegetative growth, but accumulates in the nucleus in quiescence in response to glucose starvation. By performing processes involving either drug treatment or gene knockout, they found that the phosphatase activity of Ppt1 (the co-chaperone of Hsp82) is essential for the cellular distribution of Hsp82. Ppt1 expresses at a high level under nutrient rich condition and decreases to low level when nutrient is limited. Meanwhile the nutrient-regulated expression of Ppt1 is negatively correlated to the ratio of Hsp82 nuclear accumulation. Further research showed that Ppt1 regulates the cellular distribution of Hsp82 by dephosphorylating S485 on Hsp82. They used RNA-seq to explore the physiological role of Hsp82 dynamic distribution under different nutrient conditions. RNA-seq analysis and experimental verification showed that Hsp82 is involved in the membrane-related protein transport and the regulation of cell size, thereby maintaining cell survival under starvation conditions. Mutations in the S485 residue led to a shorter survival time compared with the wild type strain. This study helps to understand the role of Hsp90 and its co-chaperone in nutrient sensing and aging process.
The study was titled “A Single Site Phosphorylation on Hsp82 Ensures Cell Survival During Starvation in Saccharomyces cerevisiae”, and was published online in the Journal of Molecular biology on September 11, 2020. Graduate student Xuan Shang and Dr. Guang Cao are the co-first authors, and Prof. Fei Dou and senior engineer Wanjie Li are the co-corresponding authors of the paper.
Original link: https://www.sciencedirect.com/science/article/pii/S0022283620305350
Highlight:
Hsp82 distributes in the cytoplasm and nucleus uniformly in nutrient-rich conditions.
Hsp82 accumulates in the nucleus under starvation and consequent quiescence.
Ppt1 dephosphorylates the S485 residue on Hsp82 in nutrient-rich conditions.
Hsp82S485A fails to accumulate in the nucleus under starvation conditions.
Ppt1-Hsp82-mediated nutrient-sensing pathway ensures cell survival during starvation.
Graphical abstract
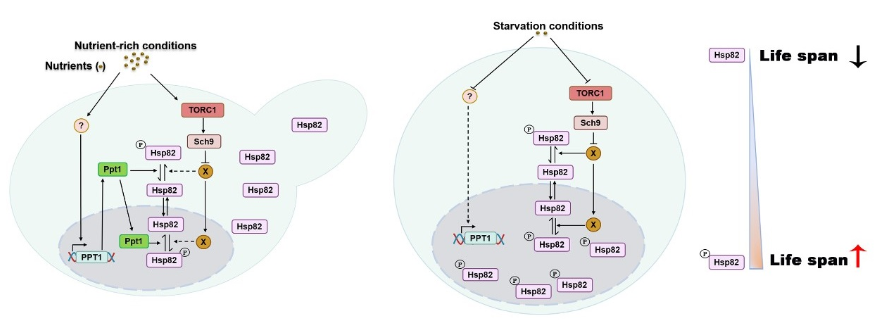
(left) Under nutrient-rich conditions, the TOR pathway maintains active, which inhibits the activity of kinase X. High-level expression of Ppt1 dephosphorylates Hsp82. Dephosphorylated Hsp82 shuttles between the cytoplasm and nucleus. (right) Under starvation conditions, Hsp82 could not be dephosphorylated by Ppt1 due to the drop-in expression of Ppt1. The kinase X is activated due to the inactivated of the TOR pathway. Kinase X phosphorylates Hsp82 causing its nuclear accumulation. Phosphorylated S485 on Hsp82 is required for the membrane-related protein transport, resistant to the cell wall drugs and heat shock and controlling of cell size. Thus, phosphorylated Hsp82 on S485 ensures cell survival under starvation conditions.


